Durban, South Africa — A lithium battery is a rechargeable product that uses lithium ions to store and release electrical energy.

Lise Oertel
Communications Specialist
What is a lithium battery?
Lithium batteries are preferred over other types of batteries because they have a high energy density. That means they store significant energy relative to their size and weight. A lithium battery has a relatively low self-discharge rate, allowing it to hold its charge for an extended time when unused.
What are the parts of a lithium battery?
The basic structure of a lithium battery consists of one or more cells, each containing a positive electrode (cathode) and a negative electrode (anode) separated by a porous material called a separator. The electrodes are typically made of materials that can intercalate lithium ions, enabling the reversible movement of ions between the two electrodes during charging and discharging.
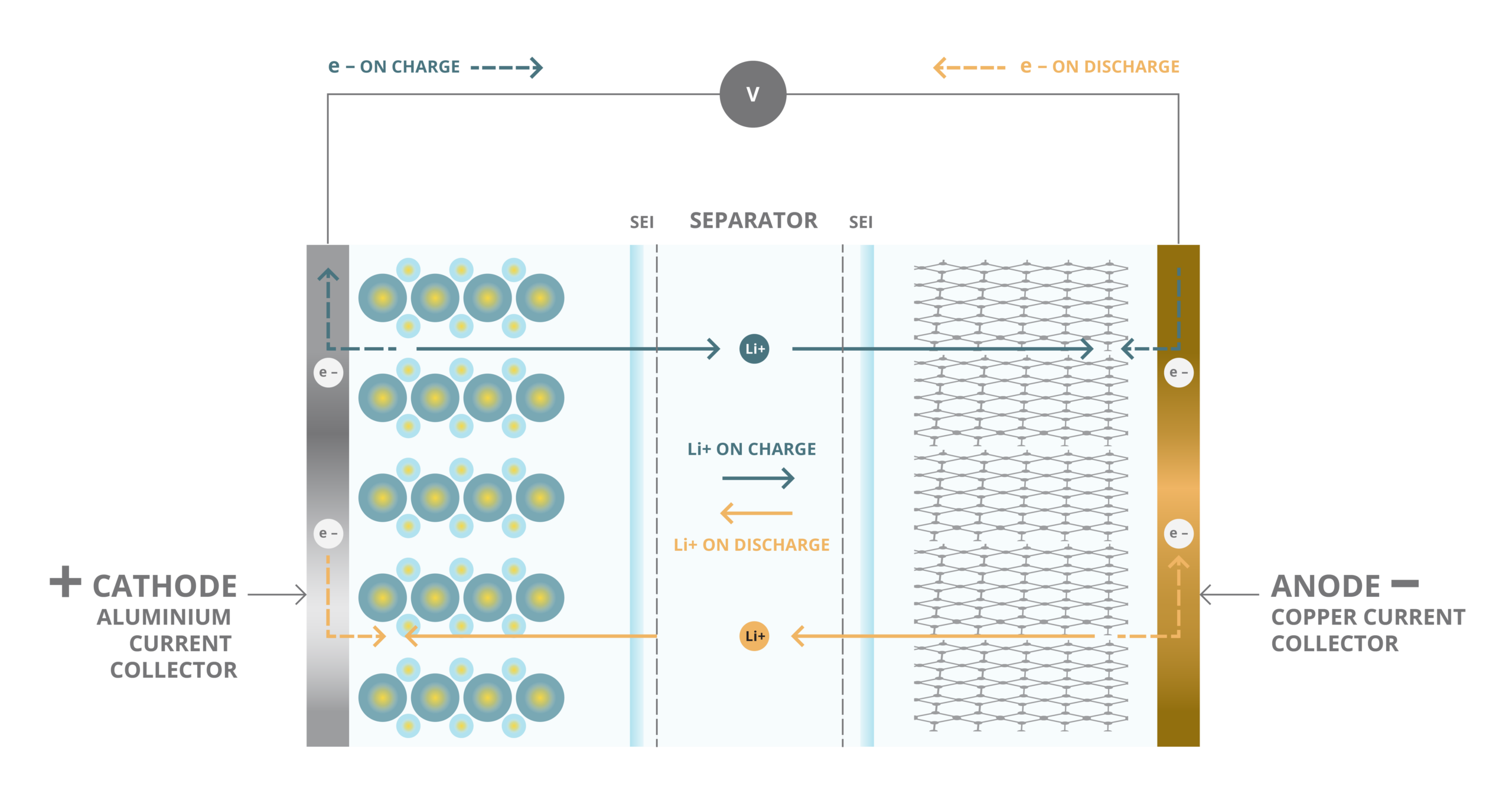
When the lithium battery charges, the ions move from the positive to the negative electrode through the electrolyte, a conductive solution containing lithium salts. This process is reversed during discharging, with the lithium ions moving back to the positive electrode, generating an electrical current that can be used to power a device.
How are lithium batteries used?
Lithium batteries are widely used in numerous portable electronic devices, such as smartphones, laptops, tablets, and digital cameras. It is a preferred power source because they provide long-lasting power in a compact form, allowing these devices to be used for extended periods without requiring another energy source.
You can also find them in electric vehicles (EVs) and renewable energy systems. The high energy density of lithium batteries achieves longer operating times and faster acceleration while having the capacity to store energy from renewable sources.
Cordless power tools like drills, saws, and impact drivers often utilise lithium batteries.
Lithium batteries are used in the aerospace and aviation industries for various purposes. They can power unmanned aerial vehicles (UAVs), satellites, space probes, and electric aircraft. The lightweight and high energy density ratings make them ideal for applications where weight and efficiency are necessary.
Lithium batteries are even found in medical devices, including pacemakers, insulin pumps, and defibrillators.
How do you extract lithium for batteries?
There are three primary sources of lithium extraction: lithium-rich brine, hard rock (pegmatite), and lithium-containing clay. Each one follows a different recovery process.
Lithium-rich brine deposits
These deposits are the most common source of lithium extraction. The brine contains high concentrations of it in underground saltwater aquifers. The extraction process typically involves the following steps.
- Brine is pumped to the surface from the underground aquifers using wells before being stored in large evaporation ponds or enclosed facilities.
- It receives weather exposure, allowing it to evaporate over several months. Various salts, including lithium carbonate, settle to the bottom of the remaining liquid.
- The concentrated lithium-rich solution is further processed to remove impurities such as magnesium, calcium, and other unwanted elements through chemical precipitation, ion exchange, and filtration.
- It undergoes additional processing to convert it into lithium carbonate or lithium hydroxide, the common forms used in battery production.
Pegmatite deposits
Hard rock deposits are a source of lithium, often found in spodumene. The area is mined using traditional techniques, with the ore extracted and sent to a processing plant.
The extracted ore gets crushed from there until it becomes a fine powder. This work increases the spodumene surface area for further processing.
Then the crushed ore undergoes gravity separation to concentrate it. This process involves techniques like froth flotation, dense media separation, or magnetic separation.
It is then roasted and given an acid treatment before being converted to lithium carbonate or hydroxide.
Lithium clay deposits
Rich clay minerals like hectorite are an emerging source of lithium extraction. After mining the material, it goes through beneficiation processes, including crushing, grinding, and flotation, to separate the lithium-bearing minerals from everything else.
After it is purified, the material is typically treated with sulfuric acid. This step extracts the lithium sulfate. It is then further processed to convert it to a usable material for battery manufacturing.
Why choose a Lithium battery over others?
Lithium batteries offer several advantages, such as high voltage, long cycle life, and low maintenance requirements. They can be sensitive to high temperatures, so storage and use considerations should be evaluated when considering this option for tools or equipment.
There is also a need for proper handling and recycling due to the presence of lithium and other potentially hazardous materials once the battery’s lifespan is over. Overall, lithium batteries have changed how the portable electronics industry operates. This power source has become vital in our shift toward electric transportation and environmentally friendly energy generation and storage.
Ongoing research and development are focused on improving each lithium battery's performance, safety, and environmental sustainability.
Related Articles
November 29, 2023
November 17, 2023